Brazil Clinical Trials Market Size, Share & Industry Analysis, By Phase (Phase I, Phase II, Phase III, and Phase IV), By Application (Oncology, CNS Disorder, Cardiology, Infectious Disease, Metabolic Disorder, Renal/Nephrology, and Others), and Country Forecast, 2025-2032
KEY MARKET INSIGHTS
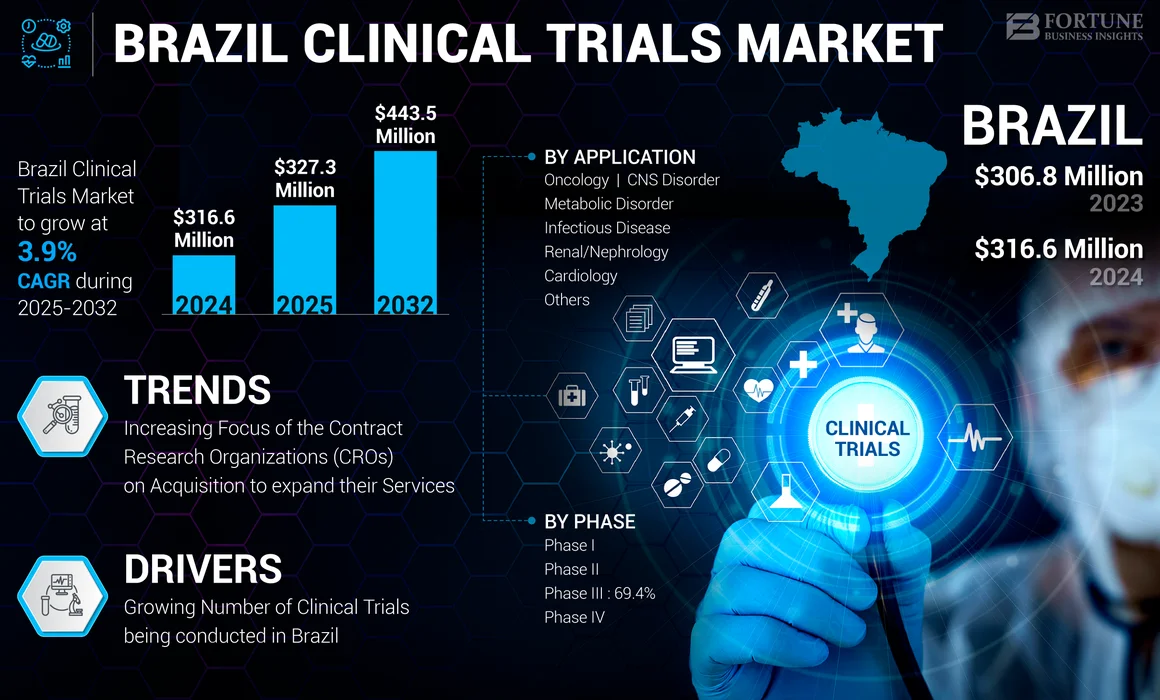
The Brazil clinical trials market size was valued at USD 316.6 million in 2024. The market is projected to grow from USD 327.3 million in 2025 to USD 443.5 million by 2032, exhibiting a CAGR of 3.9% during the forecast period.
Clinical trials are the processes that aid in researching the safety and efficacy of new treatments, therapeutics, and medical devices. The increasing prevalence of chronic diseases has been fueling the demand for effective diagnostics and therapeutics. This factor has been fueling the number of trials conducted for the development of effective therapeutics, thus contributing to the market growth.
- For instance, as per the data published by the National Center for Biotechnology Information (NCBI) in 2022, the prevalence of cardiovascular disease in Brazil was 27.5% among the total population of the country.
Moreover, the market comprises small and mid-sized players. IQVIA Inc., Laboratory Corporation of America Holdings, ICON plc, and Charles River Laboratories are a few of the key players that are enhancing their service offerings to fuel their revenue generation from clinical studies service offerings.
Market Dynamics
Market Drivers
Growing Number of Clinical Trials being Conducted in Brazil has been Fueling Market Growth
Many pharmaceutical and biotechnology companies have been focusing on the research & development of novel therapeutics to reduce the growing burden of chronic conditions majorly in Brazil.
- For instance, Genentech, Inc. initiated a phase I clinical trial in August 2022 to study the safety, pharmacokinetics, and activity of GDC-1971 in combination with atezolizumab in patients suffering from locally advanced or metastatic solid tumors. The study is estimated to be completed in March 2026. The study was conducted at 31 locations globally. Out of this, 10 locations were in Brazil.
This factor has been fueling the number of research trials being conducted in the country, thereby fueling the market growth.
- For instance, as per the data published by the World Health Organization (WHO), in Brazil, around 403 clinical studies were registered in 2024, rising from 25 in 2000.
Other Drivers
- Diverse Population: Brazil's large and varied demographic provides a rich pool of diverse clinical trial participants, which helps enhance clinical study procedures.
- Cost-Effectiveness: Conducting trials in Brazil is often more economical than in North America or Europe, thus attracting global sponsors.
- Regulatory Improvements: Recent reforms have streamlined the approval processes of clinical trials, facilitating quicker initiations.
- Enhanced Healthcare Infrastructure: Continuous advancements in medical facilities and expertise bolster the capacity to conduct sophisticated trials.
Market Restraints
Challenges Associated with limited financial and human resources in Brazil have been Limiting the Number of Trials being conducted in the Country
Factors such as a lack of financial capacity and limited human resources, among others, have been limiting Brazil clinical trials market growth.
For instance, the following are some of the major challenges faced by the biotechnology & pharmaceutical industry and research institutes while conducting clinical research in Brazil:
- Complex Regulations: The Brazilian regulatory framework is regulated by several bodies, including the National Health Monitoring Agency (ANVISA), the Brazilian Medical Association (BMA), and the Brazilian Ministry of Health (BMH). Each agency has protocols that need to be followed. These protocols and rules may change frequently.
- Approval Process: The clinical trial approval process in Brazil is lengthy and can take up to 18 months. The process includes many stages, including pre-approval, registration, and post-approval. Each step can take many months to complete.
Market Opportunities
Increasing Number of Promising Discoveries is Expected to Fuel Demand for Clinical Studies
There has been a limited number of trials and poor research and development of diseases such as oral conditions, sense organ diseases, congenital anomalies, and others due to certain barriers, such as the lack of translated human organs that limit understanding of the molecular mechanisms of disease. However, many advancements have emerged, which, with the help of additional research and funding, could lead to the discovery of advanced treatments. This can help in conducting research cost-effectively in Brazil.
Furthermore, in the coming years, new objectives are expected to emerge to treat different diseases, and studies are expected to be conducted in the sense of creating the genome of the organs affected by any disease. By improving disease knowledge and seeking the purpose of drug development, these findings will help to improve the current revival of drug development.
The recovery in the development of treatment for less-than-tested diseases, such as sense organ disorders and urinary diseases, is evident in the growing number of clinical tests, publications, and others in Brazil.
- For instance, as per the data published by the World Health Organization (WHO) in 2024, in Brazil, the number of registered research trials for sexual health conditions was 6 in 2022, whereas in 2011, there was only one clinical trial registered for the same. Similarly, for congenital anomalies, 11 trial studies were registered in Brazil in 2023, whereas 6 clinical studies were registered in 2012 for the same.
Market Challenges
- Regulatory Complexity
Navigating Brazil's complex regulatory environment requires careful planning and local expertise, which can lead to delayed initiation of clinical studies.
- Skilled Workforce Shortage
The shortage of skilled workforce in the country is among the major challenging factors in conducting trials.
Download Free sample to learn more about this report.
BRAZIL CLINICAL TRIALS MARKET TRENDS
Increasing Focus of the Contract Research Organizations (CROs) on Acquisition to expand their Services in the Country has emerged as a Latest Trend
In order to fulfill the increasing demand for pharmaceutical and other healthcare companies and aid in conducting trials in the country, CROs have increased their emphasis on the expansion of their services in the country through partnerships and acquisitions.
- For instance, in September 2022, Staphyt, a France-based healthcare service provider, acquired Phytus Group, a Brazil-based CRO company. With this acquisition, the company aimed to expand its agricultural research services in Brazil.
Other Trends
- Decentralized Trials: The implementation of remote monitoring and telemedicine to reach broader populations, including those in remote areas, has been growing significantly in the country.
- Digital Integration: The utilization of artificial intelligence and electronic consent to enhance data management and trial efficiency has been increasing in Brazil.
- Focus on Local Health Issues: Prioritizing studies for the development of cost-effective therapeutics for infectious and chronic diseases is becoming prevalent in Brazil.
IMPACT OF COVID-19
Postponement of Clinical Trials Resulted in Slow Growth of the Market During the Pandemic
During the COVID-19 outbreak in 2020, the market experienced slow growth due to the postponement of clinical studies in order to control the spread of the virus. The number of clinical trials conducted in the country also experienced a decline in 2020 as compared to the prior year.
- For instance, as per the data published by the World Health Organization (WHO), in Brazil, 1,356 trials were registered in 2020, experiencing a growth of -16.0% from the prior year.
The market grew significantly post-COVID-19 outbreak in 2021 and 2022. This was due to the upliftment of lockdown restrictions and the initiation of the postponed research studies.
SEGMENTATION ANALYSIS
By Phase
Increasing Number of Phase III Trials being Registered for Clinical Studies in the Country led to the Segment’s Dominance
The market is segmented into phase I, phase II, phase III, and phase IV, based on its phases.
The phase III segment dominated the market in 2024. The segment’s dominance is attributed to the increasing number of studies being conducted in the country.
- For instance, according to 2024 data published by the World Health Organization (WHO), it was reported that about 110 phase III clinical trial studies were performed in 2024 in Brazil.
Furthermore, the phase II segment is expected to grow at the fastest CAGR during the forecast period. This is due to the increasing focus of the pharmaceutical companies in conducting phase II clinical studies in the country.
- For instance, Alexion Pharmaceuticals, Inc. initiated a phase II clinical trial in March 2025 to study the safety and efficacy of ALXN2030 in participants with active or chronic active Antibody-Mediated Rejection (AMR). The estimated date of study completion is in April 2027. The study was conducted at 49 locations globally. Out of this, 5 locations were in Brazil.
To know how our report can help streamline your business, Speak to Analyst
By Application Analysis
Increasing Number of Clinical Trials in the Oncology Field led to the Segment’s Growth
Based on application, the market is segmented into oncology, CNS disorder, cardiology, infectious disease, metabolic disorder, renal/nephrology, and others.
The oncology segment accounted for a significant market share in 2024 and is expected to grow at the fastest CAGR during the forecast period. The segment's growth is attributed to the increasing number of clinical research studies for cancer therapeutics development.
- For instance, according to the 2024 article published by the World Health Organization (WHO), it was reported that about 50 trials were registered for malignant neoplasms in Brazil.
Moreover, the others segment dominated the market in 2024 and is expected to grow at a significant CAGR during the forecast period. The segment's growth is attributed to the growing demand for effective therapeutics for disorders such as respiratory disorders, ophthalmology-related diseases, and others, and the increasing focus of the market players on its R&D.
- In January 2025, Gossamer Bio, Inc., a clinical-stage biopharmaceutical company, presented data related to seralutinib, a treatment of Pulmonary Arterial Hypertension (PAH) and Pulmonary Hypertension Associated with Interstitial Lung Disease (PH-ILD), at the Pulmonary Vascular Research Institute (PVRI) 2025 Annual Congress in Brazil.
COMPETITIVE LANDSCAPE
KEY INDUSTRY PLAYERS
Increasing Focus of Market Players on New Service Launches is Fueling the Company's Revenue Growth
IQVIA Inc., Laboratory Corporation of America Holdings, ICON plc, and Charles River Laboratories are a few prominent key players in the market, accounting for a significant Brazil clinical trials market share in 2024. The strong presence of these companies in the market is attributed to their focus on new service launches.
- For instance, in June 2024, IQVIA Inc. announced the launch of One Home for Sites. One Home for Sites platform consolidates multiple software applications and portals related to trials into a single dashboard and sign-on. This helps clinical research sites manage their tasks and systems across all their trials effectively.
Moreover, pharmaceutical, biotechnology, and medical device companies such as Pfizer Inc., Novartis AG, and F. Hoffmann-La Roche Ltd are focusing on R&D for effective therapeutic and medical device development.
LIST OF KEY BRAZIL CLINICAL TRIALS COMPANIES PROFILED
- IQVIA Inc. (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- Parexel International (MA) Corporation (U.S.)
- Medpace Holdings, Inc. (U.S.)
- ICON plc (Ireland)
- Charles River Laboratories (U.S.)
- Synova Health (Brazil)
- H Clinical Inc. (U.S.)
- ACTIVA-CRO (Argentina)
KEY INDUSTRY DEVELOPMENTS
- January 2025 – IQVIA Inc., a provider of clinical research services, collaborated with NVIDIA with an aim to innovate IQVIA Healthcare-grade artificial intelligence (AI), enabling new levels of agentic automation of complex and time-consuming workflows across the therapeutic life cycle with the precision, scalability, and trust required by IQVIA’s customers.
- September 2024 – Parexel International (MA) Corporation, a CRO, announced the strengthening of its Real World Research (RWR) offering, bringing together the company’s Scientific Data Organization (SDO) and Real World Evidence (RWE) capabilities to meet growing customer better needs in the drug development.
- July 2024 – Charles River Laboratories collaborated with FOXG1 Research Foundation (FRF), highlighting the patient advocacy group’s model to drive drug development through the clinical phase independently. This helped the company to increase its brand presence.
- June 2024 – Charles River Laboratories entered into a partnership with Sanofi for the development of digital control groups for virtual clinical trials with an aim to avoid practice of live animal testing.
- April 2024 – Parexel International (MA) Corporation collaborated with Palantir Technologies Inc. to leverage artificial intelligence to enhance and accelerate the delivery of safe and effective clinical trials among customers. This helped the company to strengthen its presence.
REPORT COVERAGE
The Brazil clinical trials market research report provides a detailed competitive landscape and market insights. It also includes key insights, such as top industry developments covering partnerships, mergers, and acquisitions. Additionally, it focuses on key points, such as new solution launches in the market. Furthermore, the report covers regional analysis of different market segments, profiles of key market players, market trends, and the impact of COVID-19 on the market. The report consists of quantitative and qualitative insights that have contributed to the market's growth.
Request for Customization to gain extensive market insights.
Report Scope & Segmentation
|
ATTRIBUTE |
DETAILS |
|
Study Period |
2019-2032 |
|
Base Year |
2024 |
|
Estimated Year |
2025 |
|
Forecast Period |
2025-2032 |
|
Historical Period |
2019-2023 |
|
Growth Rate |
CAGR of 3.9% from 2025-2032 |
|
Unit |
Value (USD Million) |
|
Segmentation |
By Phase
|
|
By Application
|
Frequently Asked Questions
Fortune Business Insights says that the Brazil market stood at USD 316.6 million in 2024 and is projected to reach USD 443.5 million by 2032.
The market is predicted to exhibit a CAGR of 3.9% during the forecast period of 2025-2032.
By phase the phase III segment led the market.
The increasing number of clinical trials, along with improvement in the regulatory scenario, has been fueling the market growth.
IQVIA Inc., Laboratory Corporation of America Holdings, ICON plc, and Charles River Laboratories are the top players operating in the market.
Related Reports
-
US +1 833 909 2966 ( Toll Free )
-
Get In Touch With Us


