Meningococcal Vaccines Market Size, Share & Industry Analysis, By Technology (Recombinant/Conjugate/Subunit, Inactivated, and Others), By Type (MenACWY Vaccines, MenB Vaccines, MenABCWY Vaccines, and Others), By Age Group (Pediatric and Adults), By Distribution Channel (Hospital & Retail Pharmacies, Government Suppliers, and Others), and Regional Forecast, 2026-2034
KEY MARKET INSIGHTS
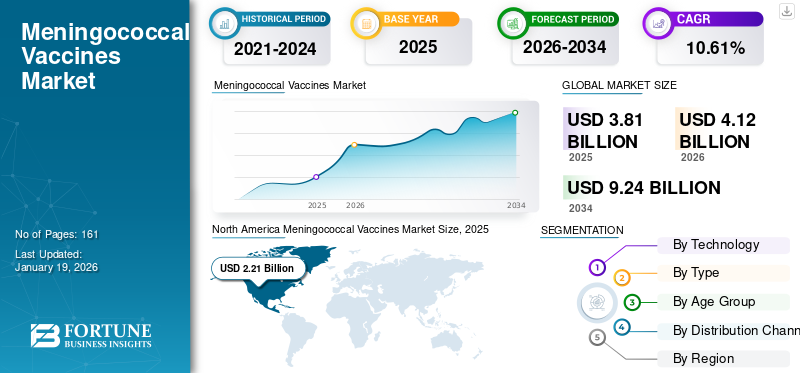
The global meningococcal vaccines market size was USD 10.61% billion in 2025. The market is projected to grow from USD 4.12 billion in 2026 to USD 9.24 billion in 2034 at a CAGR of 10.61% during the forecast period. North America dominated the meningococcal vaccines market with a market share of 13.36% in 2025.
Meningococcal diseases are a group of diseases that include meningitis, sepsis, and pneumonia, caused by infections with the bacterium Neisseria meningitidis (N. meningitidis). Meningococcal vaccines are used to protect against these infections caused by N. meningitidis. There are 12 serogroups of N. meningitidis, of which 6 (A, B, C, W, X, and Y) can cause epidemics. Bacterial meningitis is a serious infection of the thin lining surrounding the brain and spinal cord. It can cause severe brain damage and is fatal in most cases if left untreated.
Meningococcal bacteria spread through close contact, including respiratory or throat secretions from carriers. The bacteria also transmits via kissing, sneezing, coughing near someone, or sharing living spaces and utensils with an infected individual. The global market is witnessing significant growth due to the rising incidence of vaccine-preventable meningococcal infections. Additionally, increasing collaborations amongst key players and research institutes to develop new vaccine candidates is expected to boost market growth during the forecast period.
- For instance, in April 2024, Serum Institute of India Pvt. Ltd collaborated with the University of Oxford, intending to develop a chimeric protein-based vaccine to improve global access to the Men-B vaccines.
The presence of key players such as GSK plc, Sanofi, and Pfizer Inc. in the global market, with major regulatory-approved products for meningococcal vaccines, boosts the market's growth.
MARKET DYNAMICS
MARKET DRIVERS
Rising Incidence of Bacterial Meningococcal Diseases Boosts Product Demand
The increasing incidence of vaccine-preventable meningococcal disease worldwide is expected to heighten the demand for meningococcal vaccines. Meningitis is the inflammation of the tissues surrounding the brain and spinal cord. It is caused by several species of bacteria, viruses, fungi, and parasites, but bacterial meningitis is the most serious form.
- For instance, as per the World Health Organization, around 1 in 6 people who get bacterial meningitis die, and 1 in 5 suffer serious and permanent complications, including brain damage, kidney damage, hearing loss, and amputation of arms, legs, fingers, or toes.
Such severe effects of the diseases and a significant increase in meningococcal infection cases in recent years have shifted the focus toward adequate vaccination.
- For example, as per the data published by the Centers for Disease Control and Prevention, in 2023, around 438 meningococcal disease cases were reported in the U.S., the largest number of U.S. meningococcal disease cases in 10 years. Such a high number of cases increases the demand for active immunization, thus driving the global meningococcal vaccines market growth.
MARKET RESTRAINTS
High Cost of Vaccines May Restrict Market Growth
The high cost associated with the meningococcal vaccine is a significant concern globally. The high cost of the vaccines leads to decreased adoption, particularly in developing countries that face a high burden of meningococcal disease.
These high costs are primarily due to the complexity of developing and manufacturing these vaccines, especially conjugate vaccines that require specialized technology and multiple components to target different serogroups, contributing to higher production costs.
Additionally, maintaining a cold chain for vaccine transport and storage adds to logistical costs, especially in regions with inadequate infrastructure, leading to increased costs of the final products.
- For instance, as per the CDC Vaccine Price Lists posted in June 2025, Penbraya (Meningococcal (Groups A, B, C, W and Y-135)) vaccine cost around USD 230.75 for 1 pack per vial in private sector and the Menquadfi (Meningococcal Conjugate (Groups A, C, W and Y)) cost around USD 171.97 for 10 pack – 1 dose vial. Such a high cost associated with private doses can lead to decreased adoption of the vaccine among those who do not have any insurance coverage.
MARKET OPPORTUNITIES
Rising Government Initiatives for Meningococcal Vaccination Offers Lucrative Opportunities
Meningitis is epidemic across the world, particularly in sub-Saharan Africa, despite the significant availability of vaccines for meningococcal infections. Meningitis is an alarming disease worldwide with a high case fatality rate and a tendency to cause epidemics, posing a major challenge for health systems, economies, and society.
Moreover, the long-term effects of disease have an emotional, social, and financial impact on the patient and caregivers. Thus, many government organizations are launching awareness programs to increase vaccination coverage among infants, children, and adults to prevent the outbreak of vaccine-preventable diseases and reduce the financial burden on healthcare authorities.
- In April 2025, the World Health Organization issued the first-ever guidelines for meningitis diagnosis, treatment, and care to enhance the detection rate, ensure timely treatment, and improve long-term care for those affected.
- Additionally, in February 2025, under the global initiative "Defeating Meningitis by 2030”, Member States requested increased support from WHO to implement an immunization roadmap for meningitis. The support comprises immunization programs with affordable vaccines, including Men5CV, to eliminate seasonal outbreaks and protect vulnerable populations from these diseases. Such initiatives taken by government organizations aimed to increase vaccination coverage in highly affected areas and propel market growth during the forecast period.
MARKET CHALLENGES
Inadequate Storage and Logistical Challenges Lead to Potential Limitations in Market Growth
Vaccines are highly sensitive to temperature and require a cold chain during transportation and storage. However, many countries could not maintain adequate vaccination storage facilities, leading to wastage and financial losses for government bodies.
- For instance, in May 2023, as per the data published by the U.K. Health Security Agency, 2022, the value of vaccine wastage due to avoidable reasons was USD 3.3 million, including wastage caused by fridge malfunctions. Additionally, 54.0% wastage was due to unavoidable reasons, including cold chain failures caused by power cuts, amounting to around USD 3.7 million in losses.
Such losses associated with vaccine wastage increase the burden on government bodies, thus challenging market growth.
MENINGOCOCCAL VACCINES MARKET TRENDS
Pentavalent Vaccine Evolution is a Prominent Market Trend
Currently, available meningococcal vaccines do not cover all the most common disease-causing meningococcal serogroups: A, B, C, W, and Y. The monovalent A and C vaccines and the quadrivalent (ACWY) vaccines do not protect against all prevalent meningococcal strains. The five meningococcal serogroups A, B, C, W, and Y are responsible for nearly all cases of invasive meningococcal disease globally.
Thus, many key market players are focusing on developing and securing approval for pentavalent vaccines to provide maximum protection against all serogroups.
- For instance, in February 2025, GSK plc announced the approval of Penmenvy by the U.S. FDA. It is a vaccine against Meningococcal Groups A, B, C, W, and Y to protect adolescents and young adults.
Such approvals for advanced vaccines offer broader protection and simplify vaccination programs by eliminating the need for multiple vaccinations for different strains, thereby helping in a significant reduction of the meningococcal disease burden globally.
Download Free sample to learn more about this report.
SEGMENTATION ANALYSIS
By Technology
Wide Availability of Recombinant/Conjugate/Subunit Vaccines Boosted Segment's Growth
Based on technology, the market is segmented into recombinant/conjugate/subunit, inactivated, and others.
The recombinant/conjugate/subunit segment held the highest global meningococcal vaccines market share in 2024 due to the availability of various types of meningococcal vaccines in conjugate forms to protect against invasive meningococcal disease (IMD). Additionally, the increasing incidence of meningitis globally and rising government initiatives are boosting the segment’s growth in the market. Moreover, increasing new product launches of conjugate vaccines with advanced formulation by key players are expected to further boost the segment’s growth during the forecast timeframe.
- For instance, in November 2024, GSK Plc announced that the European Commission (EC) had approved a single-vial, fully liquid presentation of Menveo, where reconstitution is unnecessary before use. This Meningococcal Group A, C, W-135, and Y conjugate vaccine was developed to protect against IMD caused by bacterial serogroups A, C, W, and Y.
The inactivated segment is expected to grow with a moderate CAGR during the forecast period. The meningococcal A+C vaccine is one of the inactivated vaccines. The segment's growth is supported by its increased adoption in mass immunization campaigns during outbreaks due to meningococcus A or C.
The others segment is expected to grow with a considerable CAGR during the forecast period. The rising advancements in vaccine development technologies to develop new multivalent and combination vaccines are expected to propel the segment’s growth during 2025-2032.
By Type
Increasing Adoption of MenACWY Vaccines by Vaccination Centers Boosted Segment’s Growth
Based on type, the market is divided into MenACWY vaccines, MenB vaccines, MenABCWY vaccines, and others.
The MenACWY vaccines segment dominated the market in 2024. It is a quadrivalent vaccine developed to protect against meningococcal disease caused by four subgroups of meningococcal bacteria: A, C, W, and Y. The segment's growth is driven by its recommendation for all teenagers under school immunization programs. Additionally, increasing adoption of these vaccines in various countries propels the segment’s growth.
- For instance, in July 2025, the Vietnam Vaccine Joint Stock Company (VNVC) Vaccination Center System initiated the administration of MenACWY manufactured by Sanofi to protect against Neisseria meningitides.
The MenB vaccines segment held a substantial portion of the market and is expected to grow moderately during the forecast period. These vaccines are developed to protect against serogroup B meningococcal disease. MenB vaccines such as Bexsero and TRUMENBA are available in the U.S. Additionally, the segment's growth is attributed to the increasing approval and launch of MenB vaccines in different countries to boost adoption.
- For instance, in July 2024, GSK plc launched Bexsero in Korea after validating its immunogenicity and safety.
MenABCWY vaccines are expected to grow with a significant CAGR during the forecast period, owing to increasing research and development activities aimed at developing and launching pentavalent vaccines against meningococcal infections. Additionally, rising approvals and product launches by key companies with MenABCWY vaccines are expected to boost the segment’s growth in the market.
- For instance, in October 2023, Pfizer Inc. announced the approval of PENBRAYA (meningococcal groups A, B, C, W, and Y vaccine) by the U.S. Food and Drug Administration (FDA). It protects against the most common serogroups causing meningococcal disease in adolescents and young adults 10 through 25 years old.
By Age Group
Expansion of Vaccines Indications for Pediatrics Use to Propel Segment’s Growth
Based on age group, the market is bifurcated into pediatric and adult.
The pediatric segment is expected to dominate the global market and grow at a significant CAGR during the forecast period. Kids and teenagers are at higher risk of getting meningococcal disease. To protect against serious effects of the disease, such as infection of the bloodstream or meningitis, vaccination is recommended. Additionally, many key players are expanding their vaccine indication.
- For instance, in May 2025, Sanofi announced that the U.S. FDA had approved the expanded indication of MenQuadfi. It is a quadrivalent meningococcal vaccine, now approved for children aged 6 weeks to 23 months, for the prevention of IMD caused by Neisseria meningitidis serogroups A, C, W, and Y.
The adult segment held a considerable share of the market with rising prevalence of meningococcal diseases and increased adult risk.
- For instance, in May 2025, as per the CDC’s Meningococcal Disease Surveillance and Trends, 2024, 503 confirmed and probable cases were reported. This increase was predominantly attributed to the Neisseria meningitidis serogroup Y strain. Individuals aged 30 to 60 years, those identifying as Black or African American, and adults living with HIV were disproportionately affected. Such a rise in cases amongst adults is expected to boost the demand for vaccinations.
By Distribution Channel
Government Suppliers Segment Dominated due to its Ability to Procure Vaccines at Affordable Prices
Based on distribution channel, the market is segmented into government suppliers, hospital & retail pharmacies, and others.
The government suppliers held the largest share of the market due to the national immunization programs held by the government organizations. These initiatives help to procure and distribute vaccines to the underserved areas at affordable prices.
- For instance, in April 2024, Nigeria became the first country globally to introduce the Men5CV vaccine, recommended by the World Health Organization (WHO), and protects against five strains of the meningococcus bacteria. This initiative and emergency vaccination activities were funded by Gavi, the Vaccine Alliance, which supports lower-income countries with routine meningitis vaccinations.
The hospital & retail pharmacies segment is expected to experience significant growth during the forecast period. This can be attributed to the convenience associated with vaccination schedules and the improved availability of vaccines in remote areas, thereby enhancing vaccine distribution through these channels.
The others segment is expected to grow moderately during the forecast period due to the increasing number of private vaccination centers and hospitals with adequate facilities.
MENINGOCOCCAL VACCINES MARKET REGIONAL OUTLOOK
The global market can be segmented by region: North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa.
North America
North America Meningococcal Vaccines Market Size, 2025 (USD Billion)
To get more information on the regional analysis of this market, Download Free sample
North America’s market size stood at USD 2.03 billion in 2024 and is expected to dominate the global market during the forecast period. The rising incidence of meningococcal infectious diseases, coupled with strong healthcare infrastructure and increasing awareness programs for vaccination, is boosting the region’s growth.
- For instance, in June 2024, the Toronto Public Health (TPH) reported an increase in invasive meningococcal disease (IMD) cases. The TPH reported 13 cases higher than the total cases witnessed in any year since 2002. Such outbreaks may increase the demand for updating vaccination guidelines.
U.S.
The U.S. dominated the North American region, owing to the rising incidence of diseases and the presence of key market players engaged in research and development initiatives to propel market growth in the country.
Asia Pacific
The Asia Pacific market held the second-largest share and is estimated to grow at the highest CAGR from 2025 to 2032. The rising number of cases and the presence of key regional players with affordable product offerings augment the region’s growth. Moreover, increasing research and development and regulatory approvals for new vaccines are expected to boost the market.
- For instance, in July 2023, Serum Institute of India Pvt. Ltd. announced that the World Health Organization (WHO) had prequalified the MenFive vaccine. It is the first conjugate vaccine to protect against Africa's five predominant causes of meningococcal meningitis.
Europe
Europe is expected to hold a substantial market share owing to the presence of adequate vaccination programs with superior healthcare facilities.
- For instance, in November 2024, UKHSA data showed a significant decrease in the cases of meningococcal disease, which typically causes meningitis and septicemia. This decline was observed due to the success of the NHS vaccination programs. In 2022-23, 91.0% of children in England received 2 doses of the MenB vaccine.
Latin America and the Middle East & Africa
Latin America and the Middle East & Africa held comparatively lower shares but are expected to grow during the forecast period. The regional market growth is augmented by the increasing prevalence of meningococcal diseases and rising initiatives aimed at eliminating the disease through immunization programs, making vaccination more affordable. Such activities are expected to propel the market’s growth in the region.
- For instance, as per WHO, the largest burden of meningococcal disease occurs in an area of sub-Saharan Africa known as the “meningitis belt”. The incidence rate in the region was 0.05 per 100,000 in 2021, which gradually rose to 0.18 per 100,000 by 2023.
COMPETITIVE LANDSCAPE
KEY INDUSTRY PLAYERS
Key Players Focus on R&D to Develop New Vaccines and Expand their Share Within the Market
This market holds a consolidated structure, with players such as GSK plc, Sanofi, and Pfizer Inc. accounting for a substantial share in 2024. Their position is due to the robust product portfolios covering different strains of meningococcal bacteria. Additionally, the company has a strong focus on research and development activities to develop new vaccines, along with strategic initiatives such as collaborations, partnerships, which are further boosting their growth in the market.
Other key companies with an important presence in the global and regional markets are Serum Institute of India Pvt. Ltd., Walvax Biotechnology Co., Ltd., Bio Farma, and Hualan Biological Vaccine Inc. With their key products and strategic initiatives, these companies are expected to maintain their position in the market.
LIST OF KEY MENINGOCOCCAL VACCINES COMPANIES PROFILED
- GSK plc (U.K.)
- Sanofi (France)
- Merck & Co., Inc. (U.S.)
- Serum Institute of India Pvt. Ltd. (India)
- Novartis AG (Switzerland)
- Walvax Biotechnology Co., Ltd. (China)
- Hualan Biological Vaccine Inc. (China)
- Bio Farma (Indonesia)
- BioNet-Asia. (Thailand)
KEY INDUSTRY DEVELOPMENTS
- March 2025: Merck & Co., Inc. announced the opening of a new USD 1.00 billion, 225,000-square-foot facility dedicated to vaccine manufacturing in North Carolina.
- October 2024: GSK plc invested around USD 800.0 million to develop state-of-the-art drug substance manufacturing in the U.S. The facility was equipped with multi-purpose facilities capable of manufacturing sterile liquid vaccines and medicines.
- August 2024: Serum Institute of India Pvt Ltd. announced that the Center Drug Standard Control Organization (CDSCO) approved a Phase III clinical trial for Meningococcal (A, C, Y, W, X) polysaccharide conjugate vaccine (freeze-dried).
- July 2023: Walvax Biotechnology Co., Ltd. announced results from a Phase 4 clinical trial regarding the safety and immunogenicity of its Group ACYW135 Meningococcal Polysaccharide Vaccine compared to a licensed meningococcal conjugate vaccine of the same valence, Menactra.
- April 2020: Sanofi announced the approval of a biologics license application by the U.S.FDA for MenQuadfi meningococcal (Groups A, C, Y, W) conjugate vaccine.
REPORT COVERAGE
The global market report provides detailed industry analysis. It focuses on key market aspects, such as the epidemiology of key diseases and overview of meningococcal vaccine types. Additionally, it includes an overview of reimbursement scenarios by different countries, pipeline analysis, and vaccination coverage by key countries. Furthermore, insights on new product launches and key industry developments, such as mergers, partnerships, & acquisitions, are mentioned in the report. Besides these, it offers insights into the market trends in vaccine development.
Request for Customization to gain extensive market insights.
Report Scope & Segmentation
|
ATTRIBUTE |
DETAILS |
|
Study Period |
2021-2034 |
|
Base Year |
2025 |
|
Estimated Year |
2026 |
|
Forecast Period |
2026-2034 |
|
Historical Period |
2021-2024 |
|
Growth Rate |
CAGR of 10.61% from 2026-2034 |
|
Unit |
Value (USD billion) |
|
Segmentation |
By Technology
|
|
By Type
|
|
|
By Age Group
|
|
|
By Distribution Channel
|
|
|
By Geography
|
Frequently Asked Questions
Fortune Business Insights says that the global market size stood at USD 4.12 billion in 2026 and is projected to reach USD 9.24 billion by 2034.
In 2025, the market value of North America stood at USD 2.21 billion.
Registering a CAGR of 10.61%, the market will exhibit steady growth over the forecast period (2026-2034).
Based on technology, the recombinant/conjugate/subunit segment led the market in 2024.
The rising incidence of meningococcal infections is the major factor driving the market's growth.
GSK plc, Sanofi, and Pfizer Inc. are some of the major players in the global market.
North America is likely to dominate the market with a share of 13.36% in 2025.
Increasing regulatory approval for additional indications, government initiatives are expected to drive the adoption of the product.
Related Reports
-
US +1 833 909 2966 ( Toll Free )
-
Get In Touch With Us